views
US Human Vaccines Market: Market Overview, Key Market Segments
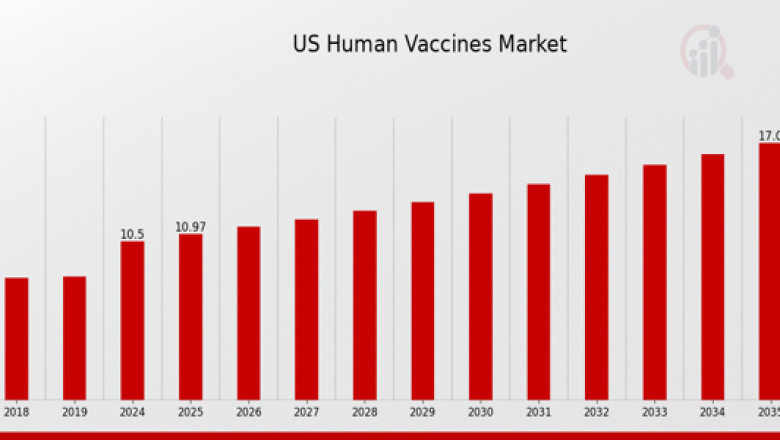
The US human vaccines market continues to be one of the most dynamic and strategically significant sectors in the healthcare industry. Valued in billions, the market has shown consistent growth, driven by the increasing demand for immunization against both emerging and existing infectious diseases. The rise in public health awareness, governmental support, and continuous technological advancements in biotechnology have significantly influenced vaccine development and delivery. The presence of major pharmaceutical and biotechnology firms in the United States has also contributed to the innovation and large-scale manufacturing of vaccines, making the country a global leader in vaccine research and production.
The US human vaccines market is segmented based on type, age group, route of administration, and distribution channel. By type, the market is categorized into preventive and therapeutic vaccines. Preventive vaccines dominate the landscape, accounting for the majority share due to their widespread use in immunization programs for diseases such as influenza, HPV, pneumococcal infections, and hepatitis. Therapeutic vaccines, although smaller in share, are gaining momentum, particularly in cancer treatment and autoimmune disorders. Based on the age group, the market is segmented into pediatric and adult vaccines. Pediatric vaccines represent a significant share, propelled by routine immunization schedules and school-entry vaccine mandates. However, adult vaccination is rapidly growing due to the increasing elderly population and the need for booster shots and vaccines for diseases like shingles and pneumococcal pneumonia. In terms of route of administration, intramuscular and subcutaneous routes are the most common, though newer technologies are pushing interest in intranasal and oral vaccines. Distribution channels include hospital pharmacies, retail pharmacies, and government supply programs, with public health programs playing a dominant role in reaching the broader population.
The US human vaccines market has witnessed several important developments in recent years. One of the most significant has been the continued investment in mRNA vaccine technology, spearheaded during the COVID-19 pandemic. Companies such as Pfizer and Moderna are extending their mRNA platforms beyond COVID-19 to include influenza, RSV (respiratory syncytial virus), and other infectious diseases. The FDA has recently approved or fast-tracked multiple new vaccines, including combination vaccines and updated COVID-19 boosters. Moreover, there has been growing emphasis on personalized vaccines and immunotherapies for cancer, which could revolutionize the therapeutic vaccine segment. Industry collaborations and public-private partnerships have also gained momentum, with joint initiatives aiming to enhance vaccine equity, improve cold-chain logistics, and accelerate research for neglected tropical diseases.
Key players in the US human vaccines market include Pfizer Inc., Moderna Inc., Merck & Co., Inc., Johnson & Johnson, GlaxoSmithKline (GSK), and Sanofi Pasteur. Pfizer and Moderna have continued to expand their mRNA vaccine portfolios, leveraging their success with COVID-19 vaccines to address other pathogens. Merck maintains a strong presence with its HPV vaccine (Gardasil) and continues to invest in oncology-based immunization strategies. Johnson & Johnson, despite setbacks with its COVID-19 vaccine, remains a significant player in the market, focusing on innovation in delivery systems and global vaccine outreach. GSK and Sanofi have diversified pipelines and continue to lead in the pediatric and combination vaccine segments. These companies are heavily investing in R&D, clinical trials, and partnerships to maintain competitiveness in an evolving market.
Several key drivers are propelling the growth of the US human vaccines market. Firstly, the increased incidence of infectious diseases and the emergence of new viral strains have highlighted the importance of vaccination as a public health tool. The recent pandemic has reinforced the critical role of vaccines in controlling disease outbreaks, thereby encouraging both governmental and private investment in vaccine infrastructure. Secondly, the aging US population has led to a surge in demand for adult vaccines, including influenza, shingles, and pneumococcal vaccines. Thirdly, strong governmental policies and funding, including support from the Centers for Disease Control and Prevention (CDC) and the Department of Health and Human Services (HHS), have bolstered immunization programs and vaccine accessibility. Additionally, advancements in vaccine technology—such as recombinant DNA technology, adjuvants, and nanoparticle delivery systems—have enabled the development of more effective and longer-lasting vaccines.
Regionally, the US human vaccines market is well-distributed but exhibits concentrated demand in highly urbanized and densely populated states such as California, New York, Texas, and Florida. These states benefit from robust healthcare infrastructure, high awareness levels, and better access to vaccination centers. However, vaccine uptake and distribution vary across states due to differing state policies, insurance coverage, and public sentiment. Rural areas face challenges in terms of logistics and vaccine hesitancy, prompting federal initiatives to bridge these gaps through mobile vaccination units, public awareness campaigns, and subsidy programs. Additionally, local outbreaks of vaccine-preventable diseases in under-immunized communities have emphasized the need for targeted vaccination efforts and improved healthcare outreach.
Explore MRFR’s Related Ongoing Coverage In Healthcare Domain:
US Oral Thin Film Drugs Market
US Pulmonary Function Testing Systems Market













Comments
0 comment