views
US Autologous Cell Therapy Market Overview
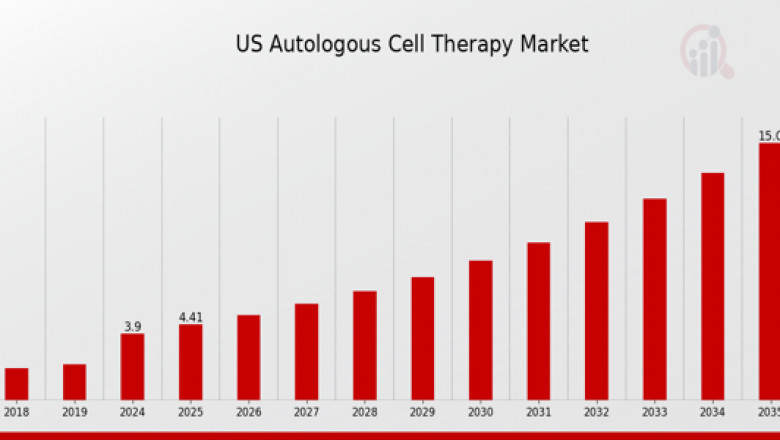
The US autologous cell therapy market is experiencing significant growth, driven by advancements in personalized medicine, increasing incidences of chronic diseases, and the growing demand for regenerative treatment options. Autologous cell therapy involves the use of a patient's own cells for therapeutic purposes, reducing the risk of immune rejection and enhancing the effectiveness of treatment. This form of therapy has garnered attention in the medical field for its potential to treat a variety of conditions, including cancer, orthopedic disorders, cardiovascular diseases, and neurodegenerative disorders. With a robust healthcare infrastructure and strong investment in research and development, the United States stands at the forefront of this transformative medical technology.
The market is segmented based on cell type, application, end user, and region. Based on cell type, the key segments include hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), chondrocytes, fibroblasts, and others. Among these, mesenchymal stem cells hold a significant share due to their versatility in treating multiple conditions such as autoimmune diseases, musculoskeletal injuries, and inflammatory disorders. In terms of application, the autologous cell therapy market is segmented into cancer, orthopedic diseases, neurological disorders, cardiovascular diseases, and wound healing. Cancer remains the largest application segment, largely due to the growing focus on developing personalized immunotherapies and targeted treatment approaches such as CAR-T cell therapies. End-user segmentation includes hospitals, clinics, and research institutes. Hospitals dominate the end-user segment owing to their advanced infrastructure, skilled personnel, and capacity for clinical trials and large-scale treatments.
Recent industry developments indicate a growing interest from both public and private sectors in autologous therapies. In 2024, several biotechnology firms and academic institutions across the United States received federal grants to accelerate clinical trials focused on autologous stem cell therapies. Moreover, strategic collaborations between pharmaceutical companies and research centers have intensified, aiming to enhance scalability, standardization, and regulatory approval for new therapies. Companies are also expanding their biomanufacturing capabilities to support the increasing number of clinical trials and product approvals. Another notable development includes the US FDA’s fast-track and breakthrough therapy designations granted to autologous therapies targeting specific cancers and rare diseases, a move that underscores the agency’s support for innovative treatment options.
Key players operating in the US autologous cell therapy market include Vericel Corporation, Lineage Cell Therapeutics, BrainStorm Cell Therapeutics, Caladrius Biosciences, Athersys Inc., and Adaptimmune Therapeutics. Vericel Corporation leads the field with its FDA-approved autologous cell therapies for cartilage repair and burn treatment. BrainStorm Cell Therapeutics has made notable progress in the development of autologous cell-based therapies for neurodegenerative conditions such as ALS. Similarly, Caladrius Biosciences is focused on autologous cell therapies for cardiovascular diseases and autoimmune disorders. These companies are investing heavily in R&D, clinical trials, and manufacturing infrastructure to stay competitive in this rapidly evolving market.
Market drivers contributing to the growth of the US autologous cell therapy sector include the rising prevalence of chronic diseases, increasing aging population, and heightened awareness about regenerative medicine. The burden of diseases like cancer, osteoarthritis, and neurodegenerative disorders is prompting patients and healthcare providers to explore innovative and long-lasting treatment options. Additionally, the preference for personalized therapies that use the patient’s own cells is increasing due to lower chances of complications and better compatibility. Technological advancements in cell processing, preservation, and delivery methods are further facilitating the development and commercialization of autologous cell therapies. Supportive government initiatives, funding for stem cell research, and regulatory frameworks that encourage innovation also serve as strong catalysts for market expansion.
Regionally, the US market benefits from strong activity in biopharma hubs such as California, Massachusetts, and Texas, which are home to numerous research institutions, biotech companies, and clinical trial centers. California remains the epicenter of innovation due to its thriving biotechnology ecosystem and academic partnerships. The Boston-Cambridge area in Massachusetts is also a leading region for autologous cell therapy research and commercialization, supported by institutions like Harvard, MIT, and the Massachusetts General Hospital. These regions are not only witnessing rapid clinical advancements but also attract significant venture capital and government support, positioning them as leaders in cell therapy innovation and development.
Explore MRFR’s Related Ongoing Coverage In Healthcare Domain:
US Oral Thin Film Drugs Market
US Pulmonary Function Testing Systems Market













Comments
0 comment