views
U.S. Duloxetine Active Pharmaceutical Ingredient (API) Market Overview
The U.S. Duloxetine Active Pharmaceutical Ingredient (API) Market is experiencing steady growth, driven by a rise in mental health awareness, increasing prevalence of depression and anxiety disorders, and growing demand for cost-effective generic medications. Duloxetine, a serotonin-norepinephrine reuptake inhibitor (SNRI), is primarily used to treat major depressive disorder (MDD), generalized anxiety disorder (GAD), fibromyalgia, and neuropathic pain. The market expansion is further supported by improvements in API manufacturing technologies and strategic collaborations among key pharmaceutical players.
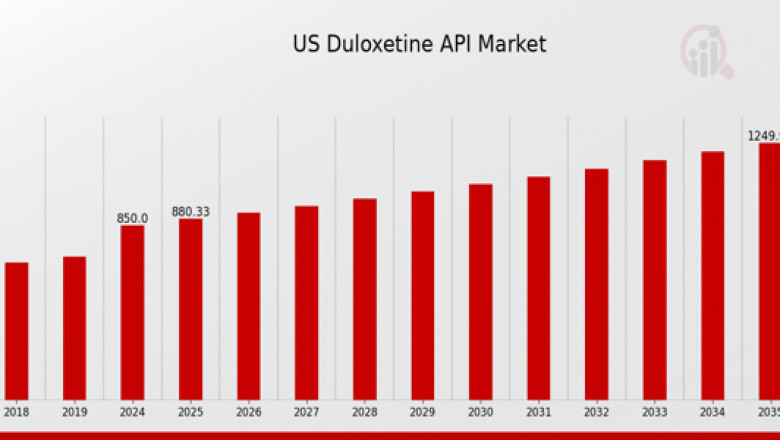
US Duloxetine API Market Industry is expected to grow from 850(USD Million) in 2024 to 1,250 (USD Million) by 2035. The US Duloxetine API Market CAGR (growth rate) is expected to be around 3.568% during the forecast period (2025 - 2035).. This growth can be largely attributed to the increasing burden of mental and chronic pain disorders, along with a growing preference for affordable generics. The expiration of patents for branded versions of duloxetine has paved the way for widespread generic drug production, improving accessibility for patients across various demographics.
Request To Free Sample of This Strategic Report - https://www.marketresearchfuture.com/sample_request/12893
The market is segmented into several key areas including application, formulation, dosage strength, and distribution channel. In terms of application, MDD and GAD dominate the market due to their high prevalence rates in the U.S. population. The drug's use in treating neuropathic pain and fibromyalgia is also expanding, especially among older adults and individuals with diabetes or chronic pain conditions. These factors contribute significantly to the ongoing demand for duloxetine-based therapies.
From a formulation perspective, the market is divided into branded and generic duloxetine products. While branded duloxetine was initially dominant, generic formulations are now leading the way due to their affordability and widespread acceptance among physicians and patients alike. The dosage strengths available in the market typically include 20 mg, 30 mg, 40 mg, and 60 mg, providing flexibility for individualized treatment regimens. Each strength addresses specific patient needs based on severity and tolerance levels, supporting comprehensive therapeutic applications.
Recent developments in the industry include the launch of new generic products and expansions of production capabilities by key players. Several pharmaceutical companies have introduced duloxetine in delayed-release capsule forms and have scaled up manufacturing to meet growing market demand. These actions not only ensure drug availability but also contribute to cost efficiency and enhanced patient adherence. Moreover, some companies are focusing on improving the bioavailability and stability of the API, aiming to enhance treatment outcomes.
Prominent companies operating in the U.S. Duloxetine API market include major pharmaceutical manufacturers such as Eli Lilly and Company—the original developer of duloxetine—as well as leading generic drug producers like Teva Pharmaceutical Industries, Sun Pharmaceutical Industries, Dr. Reddy's Laboratories, Aurobindo Pharma, and Zydus Cadila. These companies are actively investing in research and development to innovate more effective formulations, improve synthesis processes, and ensure compliance with regulatory standards. Their competitive strategies often involve partnerships, mergers, acquisitions, and licensing agreements that further strengthen market positions.
The key drivers fueling the U.S. Duloxetine API market include increasing rates of mental illness diagnoses, the aging population, and growing incidence of chronic pain disorders. Government initiatives promoting mental health awareness and generic drug usage have also played a pivotal role. As healthcare costs continue to rise, both healthcare providers and patients are seeking alternatives that offer therapeutic efficacy without the high costs associated with branded medications. Duloxetine generics fulfill this need effectively, thereby attracting more prescribers and expanding their use across various treatment plans.
Browse In-depth Market Research Report - https://www.marketresearchfuture.com/reports/us-duloxetine-api-market-12893
Regionally, the United States holds the largest share of the North American duloxetine API market. This is largely due to a strong healthcare infrastructure, high awareness of mental health issues, and the presence of multiple leading pharmaceutical firms within the country. Furthermore, favorable regulatory policies by the U.S. Food and Drug Administration (FDA) support the approval and marketing of generic drugs, thereby driving the market forward. The demand for personalized medicine and precision healthcare also contributes to the development of customized duloxetine formulations suited to patient-specific requirements.
Looking ahead, the market is poised to benefit from technological advancements in API manufacturing, including continuous manufacturing techniques, AI integration for drug development, and automation in production lines. These improvements help reduce production time and costs, ensure consistent quality, and minimize human error, further supporting the supply chain.
In addition, the increasing penetration of telehealth services and e-pharmacies in the U.S. is expected to further drive the demand for duloxetine APIs. Patients are increasingly seeking mental health consultations online and receiving prescriptions digitally, boosting the demand for accessible and affordable medications. As a result, pharmaceutical companies are adapting their supply strategies to support online platforms and ensure product availability across diverse distribution channels.













Comments
0 comment